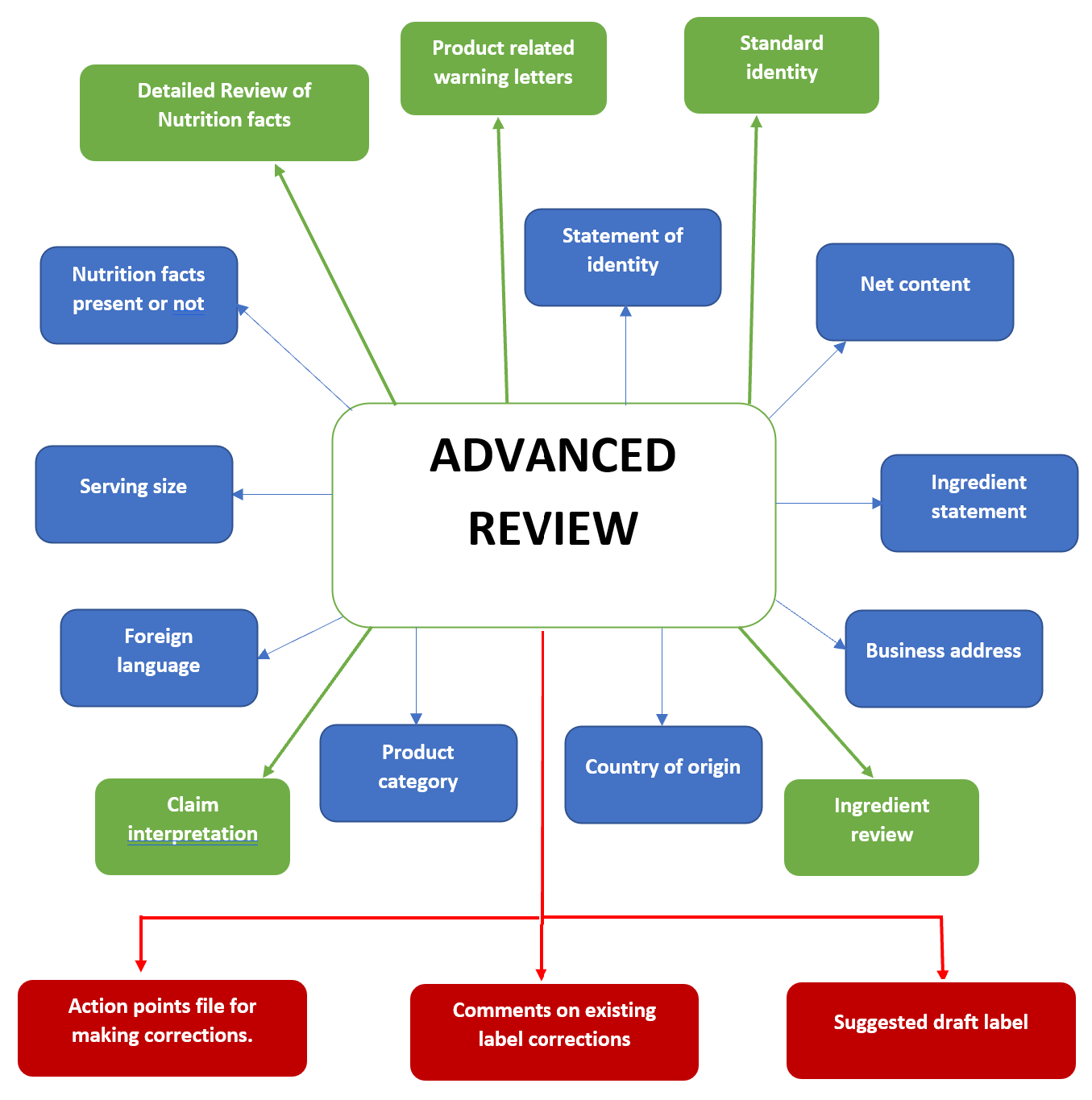
40 fda structured product labels
eCFR :: 21 CFR Part 314 -- Applications for FDA Approval to … (b) Unless provided by paragraph (d)(1)(ii) of this section, for each batch of the drug product used to conduct a bioavailability or bioequivalence study described in § 320.38 or § 320.63 of this chapter or used to conduct a primary stability study: The batch production record; the specification for each component and for the drug product; the names and addresses of the sources of the … Structured Product Labeling Resources | FDA Apr 06, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL...
The FDA-483 document with indicated PRODUCT AREA of DRUGS for: Aetna Felt Corporation 2401 W. Emaus Ave., Allentown, PA, dated: 5/13/2021-5/13/2021 2021-7096 Bridge Regulatory Affairs, LLC We ...

Fda structured product labels
Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL... Prescription Drug Labeling Resources | FDA 24.02.2022 · Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for human prescription drugs, including biological products Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics...
Fda structured product labels. Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The openFDA... Electronic Lab Notebook - ELN - Labguru Easy project & lab data management. Your experiments and lab activities generate vast amounts of research data. Labguru ELN is a secure, web-based platform to record and manage your laboratory data in one place. The electronic laboratory notebook helps you to monitor research progress and to increase its output. Scientists can design experiments and workflows with the … FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently... DailyMed 15.09.2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical …
Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics... Prescription Drug Labeling Resources | FDA 24.02.2022 · Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for human prescription drugs, including biological products Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL...







_(7396780726).jpg/510px-Looking_at_Food_Labels_(098)_(7396780726).jpg)






Post a Comment for "40 fda structured product labels"